When Dr. Jessica Little laid eyes on the 56-year-old man, Mr. M, she immediately knew he was in bad shape.
Striking, painful red welts dotted his entire arm. He knew the cause of his rashes: an infection by Mycobacterium chelonae, a bacteria that eats away at the skin and soft tissue. Yet despite multiple antibiotic treatments over six months, his arm continued to disintegrate.
Even worse, the antibacterial treatments took a toll on his body. His kidneys were damaged. The joints in his hands, wrists, knees, and ankles throbbed with arthritis. Yet the welts continued to spread.
“By the time we saw Mr. M, he had already been through a great deal,” wrote Little. “As our patient battled drug toxicities and refractory infection, we began to discuss one last option.”
The highly experimental therapy is an unassuming virus called Muddy. Originally scraped from the bottom of a rotting eggplant, the Muddy worked remarkably well. In just eight months, with the help of surgery and careful antibiotics, Mr. M’s skin rashes cleared up. Biopsies of his skin showed no sign of Mycobacteria for the first time since the initial infection.
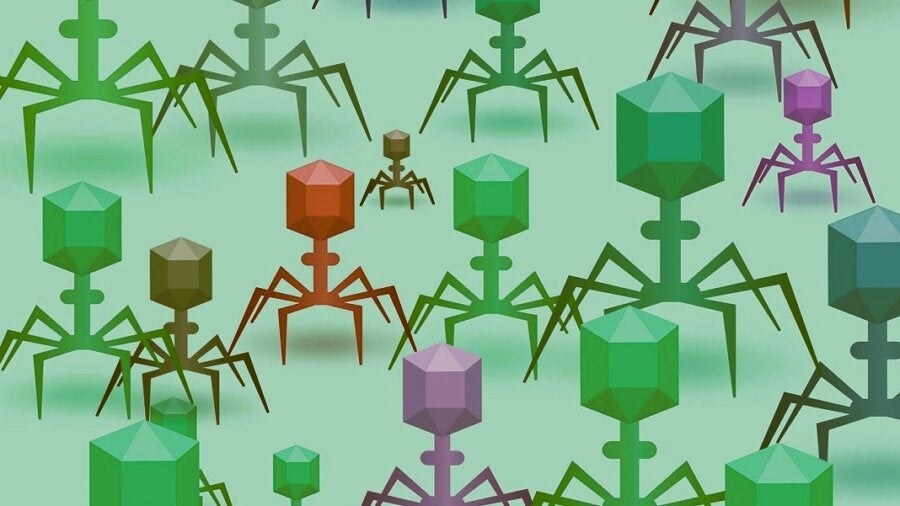
Muddy is a bacteriophage—“eater of bacteria”—a giant virus that’s a natural-born killer against even the toughest bacteria. These viruses are ubiquitous in nature. They’re sprinkled in soil, floating in our sewage, and may even be hanging out on the bottom of your shoe. Despite their lowly origins, bacteriophages may be a savior to one of the most dangerous health crises of our time: antibiotic resistance.
Bacteria Versus Phage
It’s easy to take antibiotics for granted. From treating random infections to helping ensure food security, they’re ubiquitous in our lives. Antibiotics work in different ways: they can target a phase of a bacteria’s growth, nipping the microbes in the bud, or break down their protective outer barriers.
The problem? Evolution. Bacteria are extremely well adapted to tackle a challenge through rapid gene mutations. When faced with the same antibiotic multiple times, the “stronger” ones remain, in that they’ve adapted to the effects of the drug. Even crueler, these super-powered bacteria tend to prey on the weak, such as people with languishing immune systems.
It’s a silently growing global crisis. By one estimate, in less than 30 years superbugs will kill 10 million people annually. Without new drugs to combat these deadly pathogens, we’re sitting ducks.
“We are currently facing a post-antibiotic era, in which common infections or minor injuries can become fatal,” warned Dr. Joana Azerdo, a phage therapy expert at the University of Minho in Portugal, who was not involved in the study.
Enter good ole bacteriophages. Picture a 3D hexagonal-shaped head, a long neck, and tiny spidery arms. First independently discovered by British pathologist Frederick Twort and French-Canadian microbiologist Félix d’Hérelle, bacteriophages immediately caught the medical field’s imagination as a potential ally in our ongoing war against bacteria. Evolved with bacteria, the virus seems like the perfect candidate in our fight against antibiotic resistance.
Not so fast. As viruses, phages can trigger our immune system. They’re also not a silver bullet; each phage needs to be tailored to the specific bacteria. Results of the few phage therapy trials have been inconsistent, depending on the type of infection and the health status of the patient. While generally safe, unexpected side effects, especially for a patient with an already weakened immune system, could derail the treatment.
Phage Therapy in Action
As Mr. M’s doctors debated whether to proceed with phage therapy, his condition further deteriorated, with abscesses spreading to other parts of his body.
The team pulled the trigger on the experimental treatment. The first step was to isolate the bacteria from the welts, as a target to screen bacteriophages against. They then contacted a specialized lab, led by study author Dr. Graham Hatfull at the University of Pittsburgh with expertise in Mycobacteria genetics and phage therapy. Previously, Hatfull made headlines by treating a teenager with severe mycobacterium using phages.
A molecular geneticist, Hatfull had amassed an astonishing library of phages, isolated from thousands of locations throughout the world. To narrow down potential candidates, the team honed in on roughly 20 phages that are effective against a similar bacteria. In less than a month, they found a candidate: a bacteriophage called Muddy. Isolated from the bottom of an eggplant in South Africa, the phage was highly efficient at tunneling into and killing the bacteria strain found in Mr. M’s sample.
This one phage show is quite rare, explained Little. Phage therapy is generally used as a cocktail of different types to enhance their potency. Relying on just a single strain is tricky business; if it misses the mark, then the therapy fails.
“Despite the risks of failure, he remained determined to seek any possible treatment that might improve his quality of life,” she said.
In June 2021, a year and a half after Mr. M began his medical roller-coaster ride, the doctors injected Muddy directly into his veins. Overall, the treatment was a smooth ride. With two injections daily, the man only experienced some flushing, chills, and nausea, which quickly went away. Frequent lab tests on his metabolism, liver function, and blood cell counts found everything normal. In roughly two weeks, his skin welts and rashes “improved significantly,” with “steady improvement over the subsequent months,” the authors wrote.
Can Phage Therapy Go Viral?
It’s not all rainbows and roses. In just three days, Mr. M’s immune system began generating antibodies against Muddy. By week 16, the antibody response skyrocketed, suggesting that his body may be attacking the phage therapy.
We don’t yet know why this happens. “While mounting evidence suggests a potential clinical benefit of phage therapy, many questions remain,” wrote Little. For example, it’s possible that the bacteria can adapt to phages, dwindling their bacteria-killing power. Long-term effects of phage therapy on the immune system are also a mystery, particularly for people who already have weakened immune responses. And while multiple phage therapy trials are in the works, so far most reports have been single cases—like that of Mr. M.
“Clinical trials are needed to better understand the benefits of phage therapy on a larger scale and in a more controlled setting,” said Little.
Despite the unknowns, Mr. M opted to continue his therapy, with the goal to eventually phase it out to accommodate medications for his other health troubles. As for Little, she urges the community to keep exploring phages as a therapeutic option.
Our world is littered with phages in the earth, water, and air, and crowd-sourcing programs like SEA-PHAGES (Science Education Alliance-Phage Hunters Advancing Genomics and Evolutionary Science) are helping catalog the often-overlooked viruses. Each find may be a cure for an antibiotic-resistant bacteria.
“There are an incredible number of different phages that can treat unique bacteria—this is precision medicine and it is complicated—but I believe that the development of non-antibiotic, pathogen-focused strategies to complement the tools we already have is very important at this moment,” said Little. But to get the therapy into patient hands, we need to understand the “safety, the factors driving the development of bacterial resistance” and how the body’s immune response interacts with the phages.
Image Credit: neotam / 170 images
* This article was originally published at Singularity Hub

0 Comments